Physiological function of SGLT1 & SGLT2 in the kidney
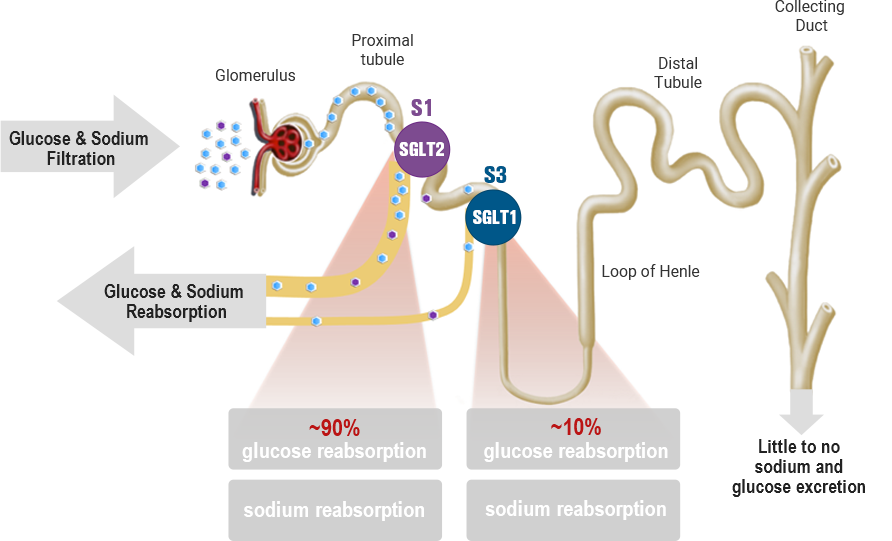
-
SGLT2 inhibition
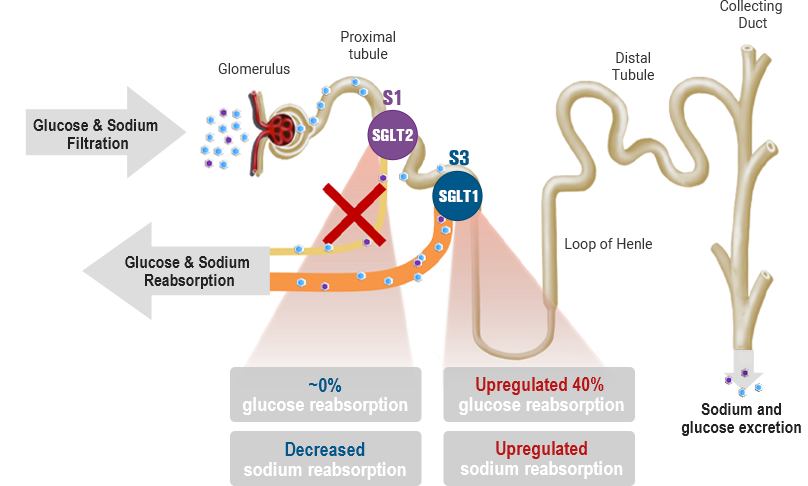
-
Dual SGLT1 & SGLT2 inhibition
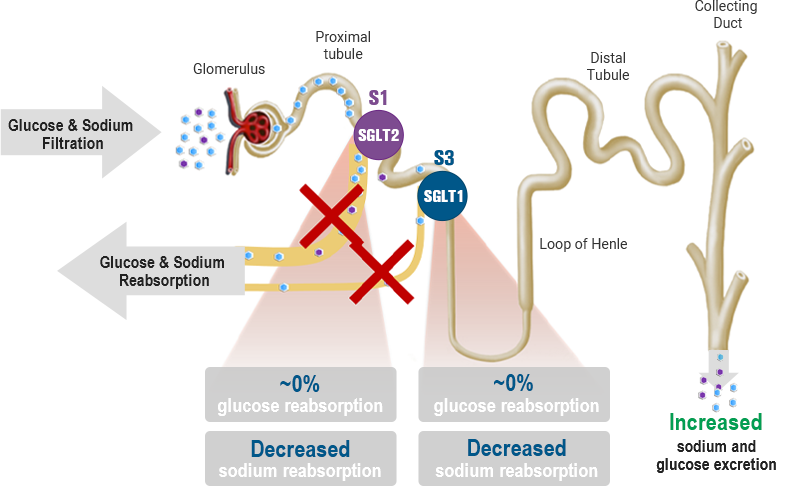
While SGLT2 is responsible for most of the filtered glucose reabsorption in the kidney, the capacity of SGLT1-mediated glucose reabsorption increases to 40-50% when SGLT2-mediated glucose reabsorption is overwhelmed or inhibited. Therefore, dual inhibition of SGLT1 and SGLT2 may serve as an important therapeutic target for a variety of diseases, including type 1 and 2 diabetes, diabetic kidney disease, and heart failure.
YG1699 is a dual, systemic SGLT1 and SGLT2 inhibitor with a potential best-in-class clinical profile. In clinical studies, YG1699 demonstrated acceptable gastrointestinal (GI) tolerability, superior post-prandial glucose (PPG) control, elevation in active GLP-1, and increased urinary glucose excretion (UGE) compared to selective SGLT2 inhibitors. YG1699 carries potential to be a superior next-generation SGLT inhibitor.
