Youngene Holds a Panel Discussion with Global Diabetes Experts at the EASD 2022 Conference
2022-12-16 10:38:52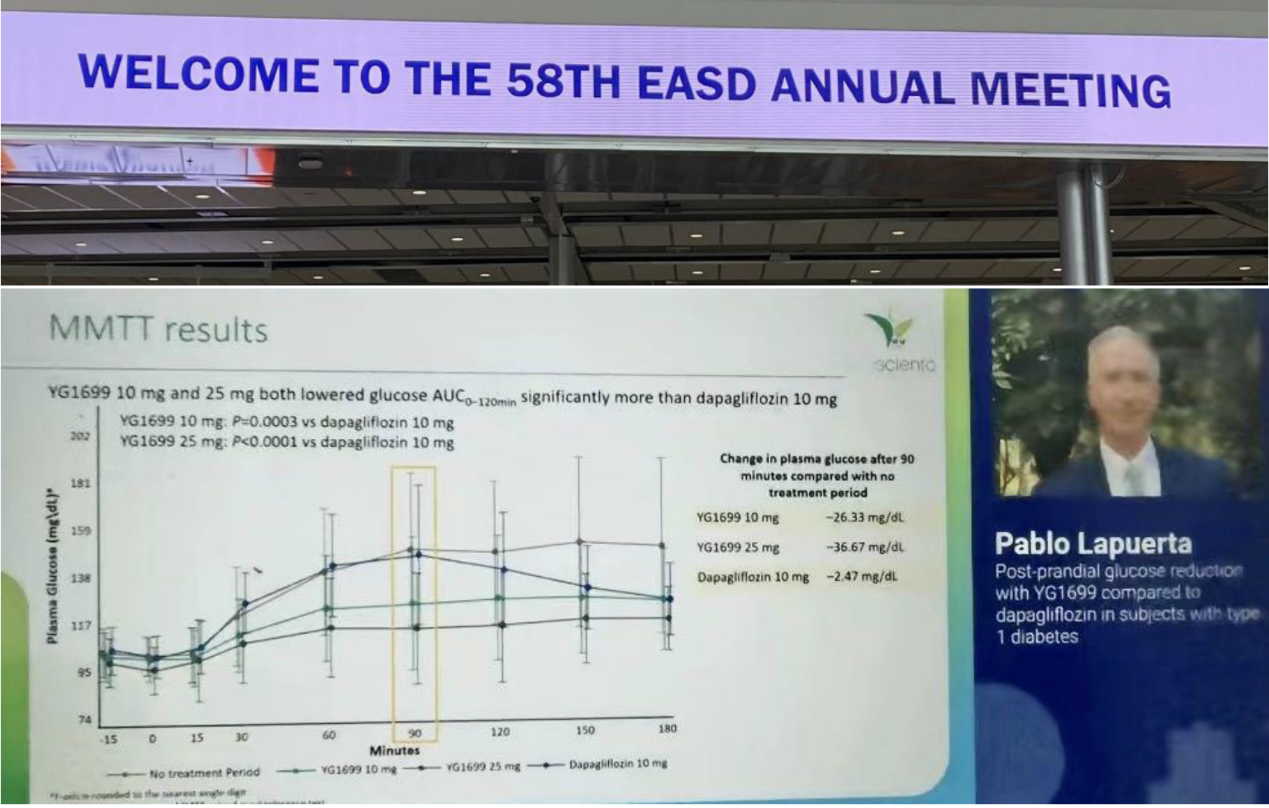
On September 21, 2022, Youngene Therapeutics’ CMO Dr. Pablo Lapuerta presented phase II clinical data of YG1699 in T1DM at the 2022 European Association for the Study of Diabetes (EASD) annual conference. On September 22, an industry expert (KOL) seminar was held. Experts gained further insight into YG1699, noting its unique and superior clinical dataset compared to dapagliflozin. Experts further commented they were delighted to hear that Youngene is among the few companies still focused on developing adjuvant therapies for T1DM and were optimistic about the compound’s prospects in type 1 diabetes, heart failure, T1DM with nephropathy, and T2DM with nephropathy.
A total of seven experts from around the world, including Europe, the United States, the United Kingdom, etc. participated in the KOL symposium. To summarize their views on YG1699:
1. The phase II clinical data of YG1699 is impressive and differentiates YG1699 in terms of its SGLT1 inhibitory activity. Such benefits can help patients and has potential to be a next-generation SGLT drug;
2. Experts recommend moving the drug forward in clinical studies of type 1 diabetes as it could be a transformative therapy for patients;
3. The nature and design of the study as a head-to-head comparison between YG1699 and dapagliflozin is important for patients with diabetes in general. Compared to dapagliflozin, YG1699 significantly improved postprandial blood sugar control, improved plasma glucose control, and endogenous GLP-1 release;
4. Recommend further studies ketone body monitoring with this class of drug;
5. Recommend strengthening cooperation with pharmaceutical companies to explore opportunities to advance the compound to larger-scale studies;
6. Remain optimistic on the potential of YG1699 in type 1 diabetes and its potentially differentiated CV-related benefits.
During the panel, experts highlighted the unmet need for patients with T1DM, referring to the lack of companies pursuing development of adjuvant therapies that carry potential to significantly reduce CV-related risks in those with T1DM.
Based on the promising features from the current dataset in type 1 diabetes patients, YG1699 carries differentiated potential in reducing heart failure and cardiovascular death. Youngene will hold further discussions with experts at the upcoming American Heart Association meeting in November 2022.