Does SGLT1 Inhibition Add to the Benefits of SGLT2 Inhibition in the Prevention and Treatment of Heart Failure?
2024-02-01 16:03:00Dr. Deepak Bhatt, a world-renowned cardiologist and advisor to Youngene Therapeutics, published a research article in the American Heart Association journal "Circulation" and the "European Heart Journal" summarizing the results of his findings of sotagliflozin as a treatment for heart failure. Sotagliflozin is the world's first marketed dual inhibitor of SGLT1/2. Currently, several selective SGLT2 inhibitors (e.g., empagliflozin and dapagliflozin) have shown significant reductions in the risk of heart failure hospitalization. Interestingly, sotagliflozin’s data demonstrate clinical superiority to currently marketed selective SGLT2 inhibitors, particularly in terms of reducing the risk of myocardial infarction and stroke. Based on the findings, the U.S. FDA broadly approved sotagliflozin as a new therapy for treatment of heart failure in May 2023.
Dr. Deepak Bhatt and his research team provided an explanation for these results. The difference likely stems from the potent SGLT1 inhibitory activity of sotagliflozin. SGLT1 is thought to promote stronger cardiac protection due to inhibition of SGLT1 and its effects on secretion of endogenous GLP-1, a mechanism that selective SGLT2 inhibitors do not possess.
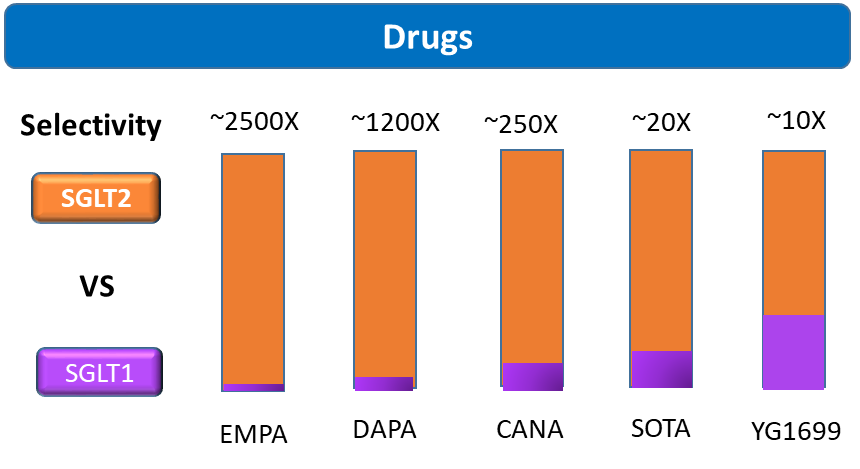
Existing data show that YG1699, Youngene’s dual SGLT1/2 inhibitor, exhibits significantly more potent inhibition activity against SGLT1 in the human body, overcoming many of sotagliflozin’s limitations as observed via pharmacokinetic (PK) data in humans. Figure 2 illustrates the proportion of SGLT1 in various inhibitors.
Paper Link:
https://www.ahajournals.org/doi/pdf/10.1161/CIRCULATIONAHA.121.054442
https://academic.oup.com/eurheartj/article/43/45/4754/6670977
Figure 1: Potential Mechanisms of SGLT1/2 Inhibitors in Cardiac Protection
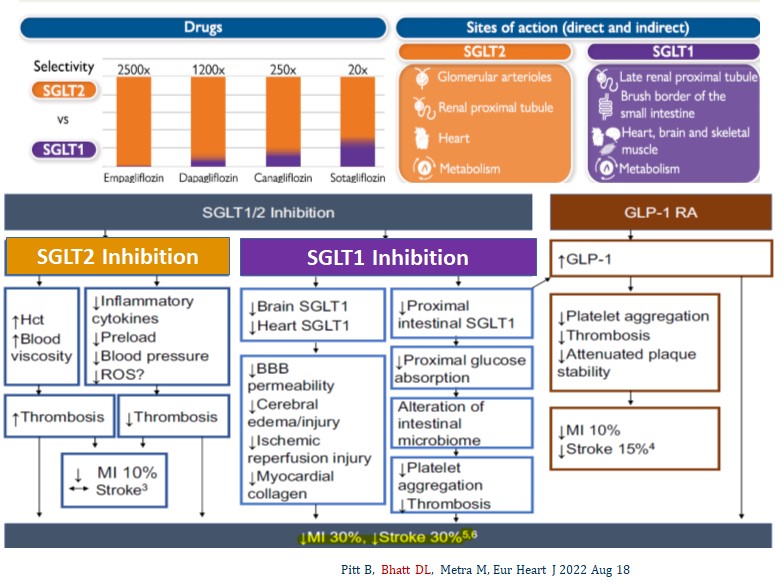
Figure 2: Comparative Schematic of SGLT1/2 Selective Data of YG1699 in Human Body
Dr. Deepak Bhatt is currently a core member of the expert advisory team at Youngene Therapeutics, and he is now actively involved in planning and developing the clinical research protocol for YG1699 in heart failure study in the United States.